

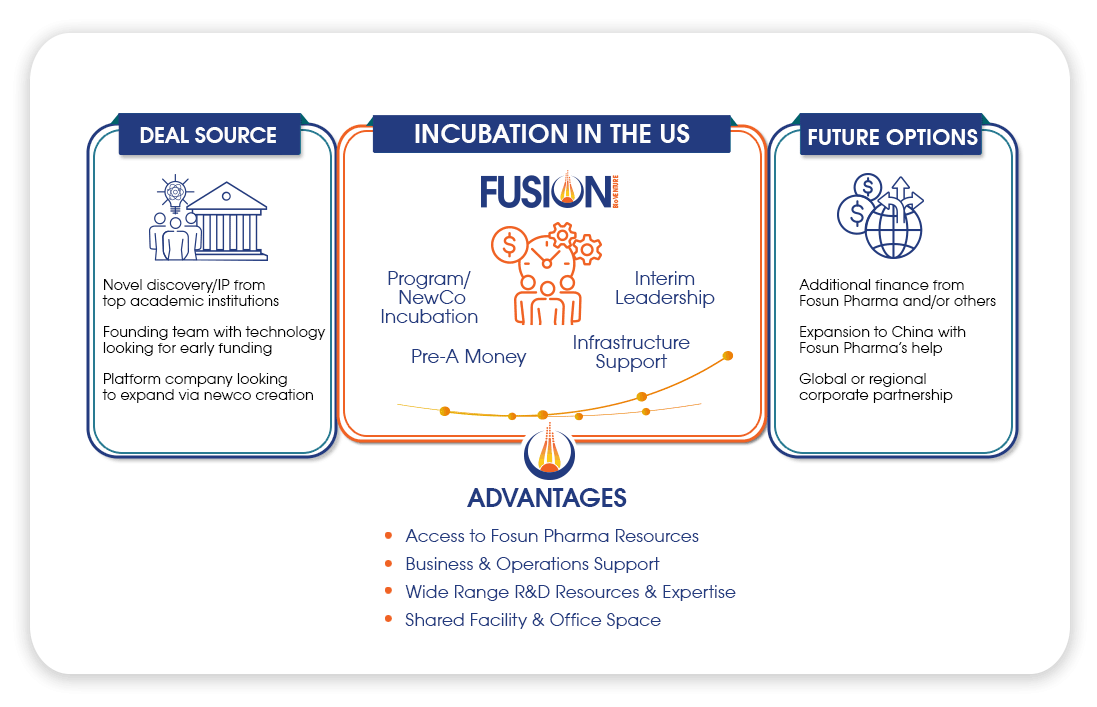
Fosun Pharma US Innovation Hub (FUSION BioVenture) is an innovation center and incubation platform to advance breakthroughs for human health.
Founded in late 2018 and based in the Boston area, FUSION has incubated and launched Archimmune Therapeutics Inc. and Leverna Therapeutics Inc., as well as licensed in two innovative programs.
Create value by incubating and investing in early-stage cutting-edge platforms and technologies


Archimmune Therapeutics was founded in 2019 by FUSION BioVenture of Fosun Pharma. Archimmune is an immuno-oncology platform company focusing on developing nanoparticle-based personalized cancer vaccines (ACNP) and multi-functional immunotherapeutics (MINP) including bispecific T-cell activators (DINP) and trispecific NK engagers (nano-TriNKE).

Leverna Therapeutics is a newco incubated by FUSION BioVenture of Fosun Pharma, with the goal of establishing a powerful RNA-targeting company. The company will be able to “drug the undruggable” by designing antisense oligos and small molecules to modify mRNA regulation. Launched in early 2022, it has a core scientific team made up of RNA biologists and plans to employ AI to help with target discovery and therapeutic design. The company currently has two programs, one for neurodegenerative disease and the other for metabolic disease.

2021-02 FUSION established partnership with Revere Pharmaceuticals to develop RAC1 small molecule inhibitor for oncology.

2022-05 FUSION established partnership with VerImmune to develop VLP-based therapeutics product in Immuno-Oncology
FUSION BioVenture
1 Hartwell Place, Suite 305
Lexington, MA 02421
Email: fusion@fosunpharma.com

104 Carnegie Center, Suite 204
Princeton, NJ 08540
(609) 250-2802
info.usa@fosunpharma.com
Customer Service & Adverse Event/ Quality Concerns: (855) 282-4882 or fosuncs@eversana.com
© 2024 Fosun Pharma USA Inc. All rights reserved. | Privacy Notice
Dr. Lijun Wu is the Chief Scientific Officer and Founder and head of FUSION BioVenture. She founded FUSION, Fosun Pharma’s innovation incubation platform in the US, which incubated and launched Archimmune Therapeutics and Leverna Therapeutics, where she is the Chair of the Board. She is also the acting CEO of Leverna. Prior to joining Fosun Pharma in 2018, she was an Entrepreneur-in-Residence at Atlas Venture and Senior Vice President of Preclinical and Clinical Development at Delinia. Prior to that, Dr. Wu held various senior leadership positions including Concert Pharmaceuticals (VP), Resolvyx Pharmaceuticals (VP), Novartis Institutes for BioMedical Research (Executive Director), and Millennium Pharmaceuticals (Director).
Dr. Wu is a seasoned executive with >25-years of experience in R&D, investment, and BD in startups established biotech, and large pharmaceutical companies in the US and across borders. She is an author of ~70 peer-reviewed publications, an inventor on a number of patents, and the recipient of Massachusetts High Tech’s “Women to Watch” award. She has been serving on the advisory council of IBBS/Johns Hopkins School of Medicine and is a member of JHU’s Society of Scholars.
Dr. Wu received her Ph.D. in Biochemistry, Molecular Biology, and Cell Biology from Northwestern University and completed a postdoctoral fellowship at Johns Hopkins University School of Medicine.
Updated 10.2023
Dr. Lijun Wu is the Chief Scientific Officer and Founder, Head of FUSION BioVenture. She founded FUSION, Fosun Pharma’s innovation incubation platform in the US, which incubated andlaunched Archimmune Therapeutics and Leverna Therapeutics, where she is the Chair of the Board. She is also the acting CEO of Leverna. Prior to joining Fosun Pharma in 2018, she was an Entrepreneur-in-Residence at Atlas Venture and Senior Vice President of Preclinical and Clinical Development at Delinia. Prior to that, Dr. Wu held various senior leadership positions including Concert Pharmaceuticals (VP), Resolvyx Pharmaceuticals (VP), Novartis Institutes for BioMedical Research (Executive Director), and Millennium Pharmaceuticals (Director).
Dr. Wu is a seasoned executive with >25-years of experience in R&D, investment, and BD in startups established biotech, and large pharmaceutical companies in the US and across borders. She is an author of ~70 peer-reviewed publications, an inventor on a number of patents, and the recipient of Massachusetts High Tech’s “Women to Watch” award. She has been serving on the advisory council of IBBS/Johns Hopkins School of Medicine and is a member of JHU’s Society of Scholars.
Dr. Wu received her Ph.D. in Biochemistry, Molecular Biology, and Cell Biology from Northwestern University and completed a postdoctoral fellowship at Johns Hopkins University School of Medicine.
Dr. Lawrence Brown serves as Associate General Counsel, Head of Legal (Fusion BioVenture) with responsibility for all aspects of the business unit’s legal affairs. He has over 20 years of experience providing advice and shaping strategies relevant to all aspects of intellectual property, litigation, commercial transactional, commercial risk, pharmaceutical regulatory, and pharmaceutical antitrust issues. Prior to joining Fusion, Larry served as Vice President and Assistant General Counsel, IP – Generics at Endo International/Par Pharmaceutical for 15 years. Before his years in-house, Larry had worked in IP litigation, IP prosecution, and biotechnology/pharmaceutical transactions at law firms in both New York City and Boston. Further, prior to his career transition to law, Larry was a postdoctoral fellow in the Biomedical Engineering Department of Boston University, continuing at that institution his graduate work in computational biophysics and molecular modeling, with a focus on protein-nucleic acid/protein-protein free energy computations, docking, molecular mechanics, and threading.
Dr. Brown holds an A.B. in Biochemical Sciences from Harvard University, a Ph.D. in molecular biology from Princeton University, and a J.D. from the Fordham University School of Law. He is admitted to the bar in New York, New Jersey, and Massachusetts as well as the United States Patent and Trademark Office, U.S. District Court for the District of New Jersey, and the Court of Appeals for the Federal Circuit. Given his scientific and legal backgrounds, Larry enjoys being at the intersection of the two, asking the “what ifs” and “why nots”, questions that are salient to Fusion’s pursuits on the cutting edge of new biotechnological solutions.
Dr. Wenwen Sha joined FUSION BioVenture in April 2022 as the Director of Biology. At FUSION, she is responsible to incubate novel platforms, building ideas into newcos, and license in cutting-edge preclinical platforms/assets for Fosun Pharma. Prior to joining FUSION, she was a Lab Head/Principal Scientist at Sanofi. Dr. Sha has extensive experience in antibody drug discovery in Immuno-Oncology, including drug discovery, target validation, and pre-IND/IND filing, with a track of record of delivering HER2/CD28xCD3 tri-specific antibodies to phase I clinic.
Dr. Sha received Ph.D. in immunology and virology from the Institute Pasteur of Shanghai, and as an exchange visitor at Baylor Institute for Immunology Research during her Ph.D. training. After graduation, she conducted her postdoctoral research in AstraZeneca/Medimmune and Cincinnati Children’s Hospital.
Valecia Holm joined FUSION BioVenture as Executive Assistant/Office Manager in 2022, supporting the day-to-day operations in areas of executive support, office management, and working on special projects across various areas of the organization.
Prior to joining FUSION BioVenture, she served as Executive Assistant/Office Manager at Erytech Pharma Inc. and had prior work experience as a Clinical Trial Associate and Supply Chain Logistics CRA. She also worked at Synta Pharmaceuticals in a similar capacity as an Executive Assistant/Office Manager.
Valecia studied Liberal Arts at Pinkerton Academy and has a 15-year background as an Ophthalmic Technician.
Dr. Linping Zhang is the SVP of Early Development for FUSION BioVenture, responsible for driving the R&D strategic planning and effective execution for FUSION BioVenture and its portfolio companies. Linping brings over twenty years of experience with biotech, pharma, and venture capital companies in driving R&D strategies, leading early development and clinical translation, conducting due diligence and in-/out-licensing in the field of oncology, ophthalmology, autoimmune and infectious diseases.
Dr. Zhang previously served as Venture Partner at 6 Dimensions Capital with major responsibility of leading clinical-stage newco incubation and launch in the immune-oncology space. Prior to that, Dr. Zhang was Executive Director and Head of Translational Sciences at BioMed Valley Discoveries, where she led and oversaw multiple cancer immunotherapy programs from IND-enabling to phase 2 clinical studies. She joined BioMed Valley from Novartis Institutes for Biomedical Research (NIBR), where she was responsible for portfolio and program management and leading multiple global cross-functional teams to advance an array of biologics and small molecule products from late research to Ph2 development for ophthalmic and oncology indications.
Dr. Zhang started her industry career at Infinity Pharmaceuticals as a founding scientist and project leader where she was instrumental in transforming the chemistry platform developed in her postdoc research lab at Harvard Medical School into the industry scale. She holds a PhD in Medicinal Chemistry from the University at Buffalo, an MBA from the MIT Sloan School of Management, and received a B.S. degree in Chemical Engineering from Tianjin University in China.
Updated 10.2023
Richard Huang joined Archimmune Therapeutics as Vice President, Head of CMC in 2023.
Richard is a seasoned pharmaceutical scientist and nanomedicine expert, bringing a wealth of knowledge and experience from both academia and industry.
Prior to joining Archimmune Therapeutics, Richard worked as the Group Head and Distinguished Scientist of non-viral gene therapy at the Genomic Medicine Unit of Sanofi. There he led groundbreaking efforts in gene replacement, gene editing, and in vivo CAR-T. Prior to that, he served as the Vice President of Process Sciences and Manufacturing at LEAF Pharmaceuticals and LEAF4Life Inc., during which he developed the formulation and process for four IND enabling GLP tox study products and achieved an emergency use approval in France for a COVID-19 related ARDS treatment.
Before these impactful experiences, Richard held various roles at Merrimack Pharmaceuticals for over nine years, leading efforts in liposome formulation, process development, and GMP manufacturing. Notably, they received approval for liposomal irinotecan (Onivyde) for the treatment of metastatic pancreatic cancer during his tenure.
Before transitioning into the industry, Richard spent nearly eight years at the University of California, San Francisco, focusing on advanced drug delivery systems using polymers and lipids. During this time, he worked on the development of a series of sterol-modified lipids, significantly advancing stable liposome formulation.
Richard holds a Ph.D. in Polymer Sciences from Fudan University and a BS in Chemistry from Wuhan University. Additionally, he is both a CMC Certified Professional and a Pharmaceutical Development Certified Professional. His dedication to furthering knowledge in his field is evident in his over 30 peer-reviewed scientific papers and numerous patents.
Updated 10.2023
Dr. Hong Ren joined Archimmune Therapeutics in June 2022 as Head of Pharmacology, responsible for preclinical pharmacology and translational research studies in support of Archimmune’s nanoparticle-based immunotherapeutics development.
Prior to joining Archimmune, Dr. Ren served as the Program Lead for multiple therapeutic antibody programs at Bluefin Biomedicine and Dynamicure Biotechnology, including antibody-drug conjugate and immune checkpoint inhibitors. She has over 15 years of experience in antibody drug discovery from concept to IND-enabling studies, focusing on preclinical biology and MOA studies for drug candidates in oncology and immuno-oncology.
Dr. Ren received a BS in Biology from Wuhan University and a Ph.D. in immunology from the University of Texas Southwestern Medical Center in Dallas. She conducted postdoctoral research at Harvard Medical School with Dr. Klaus Rajewsky.
Dr. Xiyu Ke joined Archimmune Therapeutics as a Senior Scientist, in Immuno-oncology, focusing on developing nanoparticle systems for cancer immunotherapy.
Prior to joining Archimmune Therapeutics, Xiyu worked as a Scientist at GreenLight Biosciences, designing and developing lipid nanoparticles for mRNA vaccine delivery.
Xiyu holds a Ph.D. in Nanomedicine and Biomaterials from the National University of Singapore. He completed his postdoctoral training at Johns Hopkins University. His research focuses on designing and developing functionalized nanoparticle systems for various biomedical applications, including cancer immunotherapy, cancer chemotherapy, mRNA vaccine, and antimicrobial polymers.
Updated 10.2023
Dr. Avery (Qinghong) Yan is the Co-Founder, President, and CSO of Leverna Therapeutics, a Boston-based startup company focusing on developing antisense oligonucleotides and small molecules for RNA targeting. Avery was the Head of Biology at FUSION BioVenture of Fosun Pharma USA. Prior to joining FUSION Bioventure in 2020, she was the Cell and Molecular Biology team lead at the Translational Systems Biology group at Amgen.
Avery obtained her Ph.D. in Neuroscience at Stony Brook University and completed her postdoctoral research at Columbia University.
Avery has extensive experience in R&D, investment, business development, and start-up incubation in biotech and pharma companies. Her drug development experience includes a broad range of modalities, including small/large molecule, ASO, siRNA, CAR-T, TCR-T and PROTAC programs. She has a track record of leading projects, closing deals, attracting talents and building teams.
At Amgen, Avery led the predictive and mechanistic toxicology research and supported over 15 projects with 5 IND filings. At FUSION BioVenture, she led license-in of preclinical assets into Fosun Pharma and the incubation of a US-based newco (later Leverna). In 2022, Avery started running Leverna full time with funding from Fosun Pharma USA and established the company as an RNA-targeting platform company with multiple assets in CNS and related disease areas.
Avery is also the co-founder and President of BioSpark Group, a non-profit organization based in the Boston area, focusing on establishing a biotechnology ecosystem with a collaborative and supportive scholar network, promoting leadership and entrepreneurship, and facilitating the translation of scientific discoveries in life sciences.
Dr. Zhou Han joined the Leverna Therapeutics team in 2023 to lead the group’s preclinical drug discovery and development programs for CNS diseases. Prior to joining Leverna Therapeutics, Dr. Han was a Senior Principal Scientist at Stoke Therapeutics, developing antisense oligonucleotide therapeutics for rare genetic disorders. Prior to joining Stoke Therapeutics, he studied HCV drug resistance at Bristol Myers Squibb.
Dr. Han obtained his Ph.D. in Neuroscience at the University of Connecticut Health Center, studying photoreceptor cell biology. He received his postdoctoral training at Yale University and developed novel genetically encoded voltage indicators useful for all-optical electrophysiology.
Dr. Chunsu Xu joined Leverna Therapeutic in January 2022 as a biology lead for target and disease review, and competitive landscape analysis. Before joining Leverna, she worked for the FUSION team as a scientific analyst. She covered technology platforms including RNA therapeutics, LNP delivery technologies, epigenetic therapeutics, RNA editing, etc.
Dr.Xu obtained her Ph.D. in neuroscience from Stony Brook University. Her Ph.D. study combined genetic, imaging, and behavior methods dissecting disease mutations’ contribution to memory at Cold Spring Harbor Laboratory, NY, USA. After her Ph.D., she worked as a research fellow at Novartis’s Friedrich MIescher Institute (Basel, Switzerland) for CNS disease research.
Dr. Binkai Chi joined the Leverna Therapeutics team in 2023 as a Senior Scientist. Dr. Chi has extensive experience in RNA biology and cell biology, primarily RNA binding proteins and their roles in neurodegenerative diseases. Prior to joining Leverna Therapeutics, Dr. Chi was serving as the Postdoctoral Research Associate at Dr. Reed’s lab at Harvard Medical School, using gene-edited ESC/iPSCs to study ALS.
Dr. Chi received a Ph.D. degree in Biochemistry and Molecular Biology from the Shanghai Institute of Biochemistry and Cell Biology, where he studied cellular and viral mRNA transport mechanisms.